The UK has launched a world-first clinical trial to see whether a booster vaccine dose could protect people against Covid-19 and its variants.
Seven existing vaccines are to be tested in the Cov-Boost trial to see which jabs could be used in any forthcoming autumn vaccination programme.
Some 2,886 people aged 30 and older are being recruited at 18 NHS sites from London to Glasgow, with the first booster jabs administered in early June.
Scientists want people who received their first dose of either Pfizer/BioNTech or AstraZeneca in December or January to sign up, and hope people aged 75 and over will also come forward.
Experts believe that all seven vaccines will boost immunity, and lab studies will check their response to variants circulating in the UK, including those from India, Kent and South Africa.
The £19.3 million clinical trial will test the Pfizer jab alongside those from AstraZeneca, Moderna, Novavax, Janssen from Johnson & Johnson, Valneva and CureVac.
Three of the vaccines will also be tested at a half dose, with experts expecting an adequate immune response at this level.
The half doses will inform whether side-effects are reduced at a lower dose, and could offer useful information to countries where vaccine supply may be more scarce.
The 18 NHS sites across the UK will be split into three groups, with each group testing a different set of vaccines.
All of the information will be fed to to the Joint Committee on Vaccination and Immunisation (JCVI) at the end of August or early September.
The JCVI will then guide the Government on whether people should be boosted with a third dose and which vaccines should be used, depending on supply.
The 18 sites include Southampton, London, Leicester, Bournemouth, Portsmouth, Wrexham, Bradford, Oxford, Glasgow, Leeds, Cambridge, Birmingham, Brighton, Stockport, Liverpool and Exeter.
Among the information gathered will be any data on side-effects, including among people whose third booster jab is a different type to that used for their first two shots.
Professor Saul Faust, director of the National Institute for Health Research Southampton clinical research facility and lead investigator for the trial, said the “hope of a booster is that we raise the antibody level enough to be able to cover existing and variant strains of coronavirus.”
He added: “We’re hoping the immune responses will be high enough to protect people against all the strains circulating in the UK, including we’ll be testing in the lab against the Indian variant, the South African variant, the Kent variant as well as the original strain.”
Any other variants that England’s chief medical officer, Professor Chris Whitty, wants adding to the mix can be tested as part of the trial over the summer.
Experts believe booster shots of existing vaccines could be enough to provide protection against all variants, with some scientists suggesting that developing new vaccines against variant strains may actually impair people’s immune responses.
Dr Matthew Snape, associate professor of paediatrics and vaccinology at the University of Oxford, told a briefing that changing the vaccine to, for example, one that targets the South African variant, could actually leave the body trying to respond to the original Wuhan strain of coronavirus that an earlier vaccine protects against.
He said more research was needed, but added: “In some situations you never forget your first love…you’re still trying to respond to that first vaccine.”
The researchers stressed that the aim of the new study is not to pit the vaccines against one another, but to check whether they all increase antibodies and to look for potential side-effects.
All participants in the trial will have bloods taken to measure their immune responses at days 28, 84, 308 and 365 of the trial – with a small number having blood tests at other times.
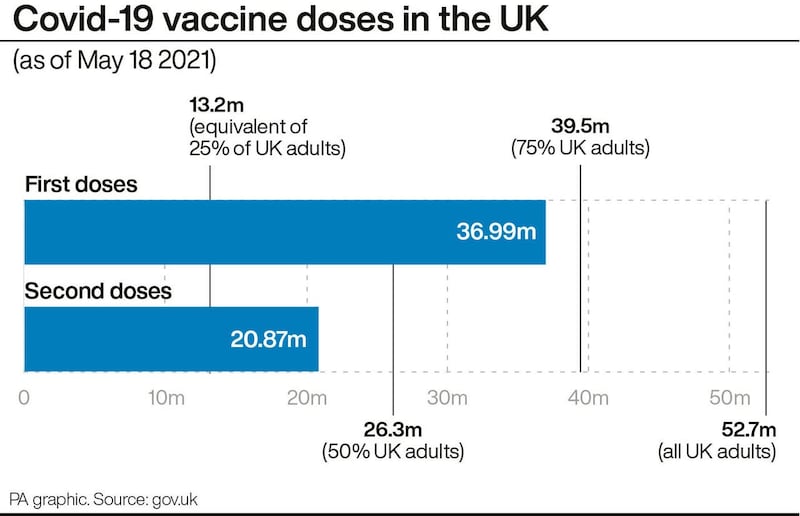
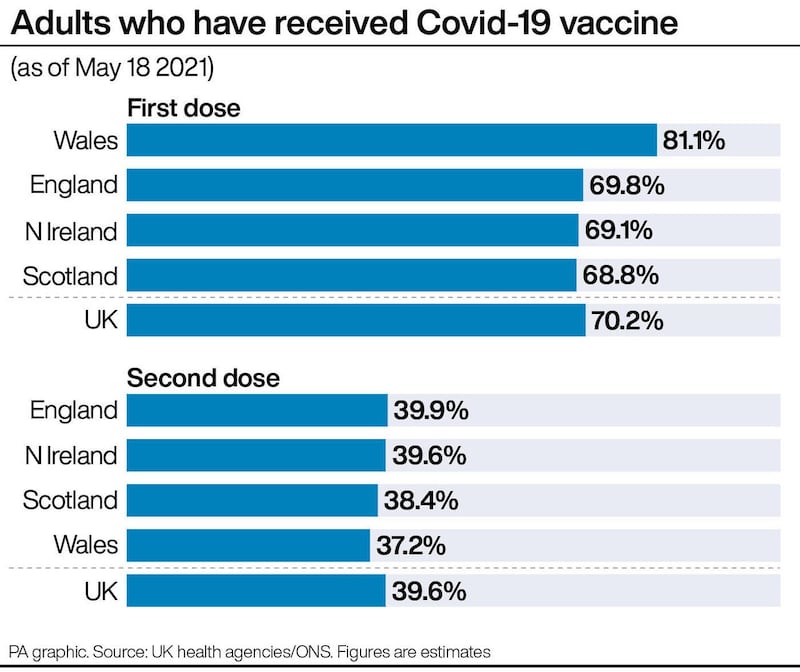
Health and Social Care Secretary Matt Hancock said: “The UK vaccination programme has been a phenomenal national effort, with seven in 10 UK adults now having had their first Covid-19 jab.
“It is vital that we continue to support the world-renowned British research sector that has contributed to its success.
“We will do everything we can to future-proof this country from pandemics and other threats to our health security, and the data from this world-first clinical trial will help shape the plans for our booster programme later this year.
“I urge everyone who has had both doses of a Covid-19 vaccine, and is eligible, to sign up for this study and play a part in protecting the most vulnerable people in this country and around the world for months and years to come.”
Prof Faust added: “This trial will give the Joint Committee on Vaccination and Immunisation the important data to inform their recommendations of how to protect the population against any future wave.
“It is fantastic that so many people across the country have taken part in vaccine trials up to now so that we can be in a position to study the effects of boosters, and we hope that as many people as possible over the age of 30 who received their first dose early in the NHS programme will be able to take part.”
Vaccines minister Nadhim Zahawi said: “Having taken part in a Covid-19 vaccine clinical trial myself, I would encourage everyone eligible to volunteer – whatever your religion, ethnicity or background.”
“It’s a fantastic opportunity to get involved with such an historic initiative.”
Further results from the ComCov clinical trial, which aims to determine the effects of using different vaccines for the first and second dose, are due in the coming months.
People can sign up for the new trial at covboost.org.uk







